New product line extension for the HydroMARK™ breast biopsy site markers
April 14, 2021
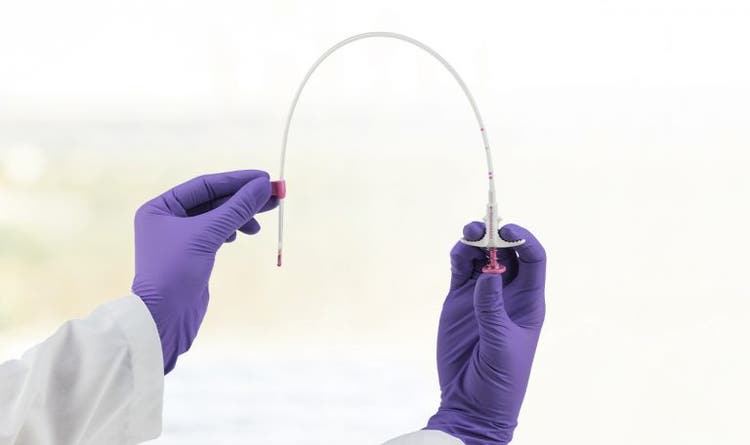
Devicor Medical Products, Inc. (Mammotome) Announces a New Product Line Extension for the HydroMARK™ Breast Biopsy Site Markers
CINCINNATI, OHIO – April 14, 2021 – Mammotome is pleased to announce a new product line of flexible HydroMARK™ Breast Biopsy Site Marker applicators designed to make the clinician’s user experience easier and more intuitive. The new line of applicators expands compatibility with leading vacuum-assisted breast biopsy systems, offering the HydroMARK™ markers as a solution to more customers, in more clinical situations.
The exclusive hydrogel technology, found in the HydroMARK™ marker line, hydrates reaching greater than 90% water in less than 24 hours.1,2,3 This enables long-term ultrasound visibility, even in patients undergoing Neoadjuvant Chemotherapy.2,3 The expanded use of biopsy marker shapes in the HydroMARK™ marker portfolio, optimizes patient follow-up care, providing clinicians three unique shape options to better track distinct biopsy sites.
The new flexible applicator design allows for easier deployment in upright biopsies. In addition, it expands compatibility with leading vacuum-assisted biopsy devices on the market today, including the Mammotome Revolve™ Dual Vacuum-Assisted Breast Biopsy System. “The ability to bend the cannula allows me to insert the flexible HydroMARK™ marker in the tight areas we often encounter with stereotactic biopsies creating a more efficient procedure and better experience for me and my patients,” said Dr. Meghan Musser of Kettering Medical Sycamore Center.
As the global market leader in breast biopsy markers, Mammotome is committed to providing customizable breast marking technologies, designed to meet clinicians’ needs and the individual needs of their patients.
“We are very excited about the addition of the flexible HydroMARK™ markers to our product portfolio,” said Michael Feld, President of Mammotome. “The launch of the new HydroMARK™ applicators not only improves the user experience, but it provides more opportunities for clinicians to utilize this unique technology that enables long-term ultrasound visibility.”
Mammotome continues to focus on delivering cutting-edge breast care solutions that improve both the physician and patient experience. After receiving positive feedback from early markets, the new flexible HydroMARK™ applicators are now available to clinicians in the U.S. with plans to expand to other regions in the near future including Canada, Japan and other countries worldwide.
Media Contact: Erica Bednar, Director of Marketing Communications
Phone: 513-391-4737
[email protected]
About Mammotome
At Mammotome, our expertise and compassion for breast care makes us the indispensable partner to physicians, clinicians and patients. Our drive for innovation is rivaled only by our compassion for the people we serve, from the clinicians and surgeons who demand consistently precise solutions, to the patients and families seeking peace of mind. We boast a comprehensive range of products that create better outcomes in breast care and provide physicians and patients with educational resources that guide their journey. Headquartered in Cincinnati, OH, Mammotome has been part of the Danaher Corporation since 2014. The Mammotome brand of products is sold in over 50 different countries throughout the world.
###
1. Sakamoto, N., Fukuma, E., Tsunoda, Y. et al. Evaluation of the dislocation and long-term sonographic detectability of a hydrogel-based breast biopsy site marker. Breast Cancer 25, 575–582 (2018). https://doi.org/10.1007/s12282-018-0854-8
2. Pinkney, Journal of Diagnostic Medical Sonography 29(4) 151 – 158, DOI 10.1177/8756479313486962
3. Blumencranz, Ann. Surg. Oncol. (2014) 21:3273-3277, DOI 10.1245/s10434-014-3917-x
©2021 Devicor Medical Products, Inc. Mammotome and HydroMARK are registered trademarks of Devicor Medical Products, Inc., in the USA and optionally in other countries.