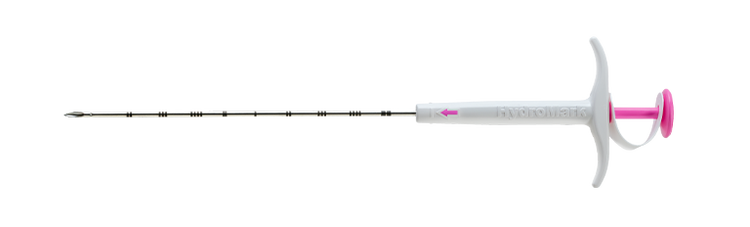
HydroMARK™ Plus
Breast Biopsy Site Marker
Same great benefits of the original HydroMARK™ markers, with added features designed to alleviate displacement, enhance visibility, and ease of locating.1, 2, 3
HydroMARK™ Plus Breast Biopsy Site Marker
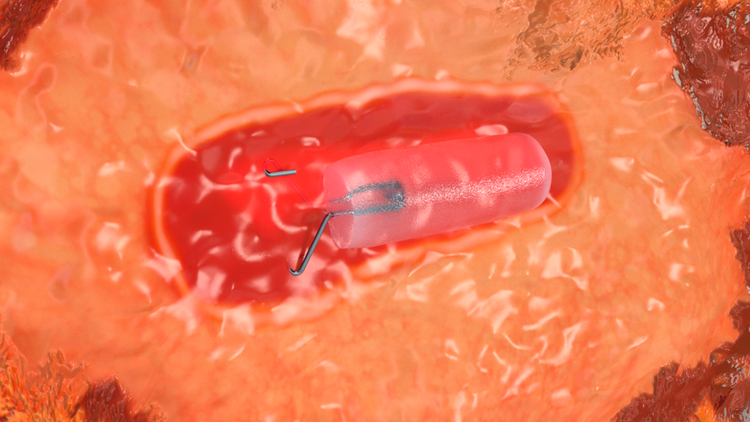
Attachment to Tissue
“Wings” are designed to anchor to the tissue to mitigate displacement during surgical excision.2

Enhanced Distinguishability
The Dragonfly™ and Hummingbird™ clips are distinct and discernably man-made to breast anatomy.

Ease of Locating
A portion of the clip is outside of the hydrogel offering additional echogenicity.
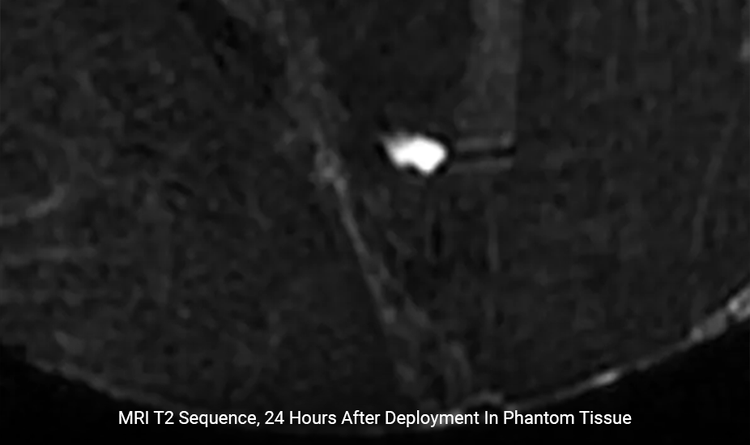
Minimal Artifact
Artifact is 1 x 1mm under Spin Echo and 3 x 3mm under Gradient Echo.4
Two unique shapes with "wings" designed to attach to breast tissue
- Dragonfly™ Clip
- Hummingbird™ Clip


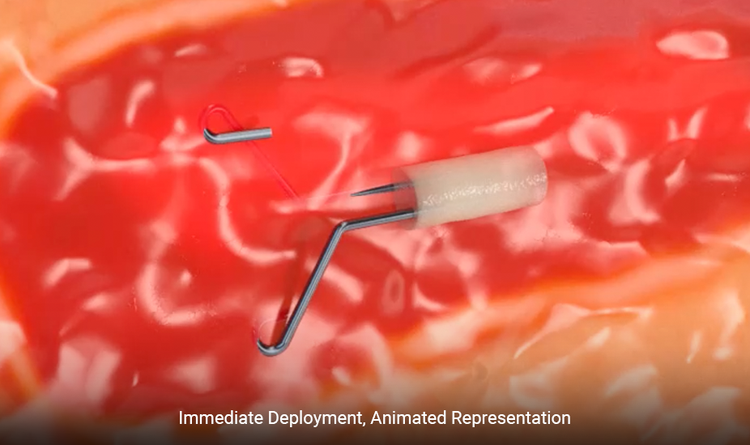
“Under ultrasound, the HydroMARK™ Plus marker is clearly visible as parallel hyperechoic wings, easily differentiated from other clips in the breast. This unique shape allows rapid detection in the operating room, improving efficiency and intraoperative localization.”
Priya Jadeja, MD, FACS
Breast Surgery, Oncology, Florham Park, New Jersey
Frequently Asked Questions
What is the composition of the hydrogel?
The hydrogel is composed of polyethylene glycol (PEG).
Do the HydroMARK™ Plus markers contain nickel?
The HydroMARK™ Plus titanium markers do not contain nickel.
What is the duration of the HydroMARK™ Plus marker’s enhanced long-term visibility?
Studies have shown that visibility can last up to 12 months in some patients.5
Can you use the HydroMARK™ Plus markers in lymph nodes?
The HydroMARK™ direct puncture applicators are indicated to mark tissue during a percutaneous breast biopsy procedure, including axillary lymph nodes.6
What size are the HydroMARK™ Plus clips?
The Dragonfly™ and Hummingbird™ clips are both 6.35mm (0.25”) x 5.08mm (0.2”), not including the hydrogel component.
How much of the marker extends outside of the hydrogel?
The bare portion of the marker outside of the hydrogel may vary in length depending on hydration, up to 3mm (0.12”).
Related Products

HydroMARK™ Breast Biopsy Site Marker
Enduring exclusive hydrogel technology provides long-term ultrasound visibility in percutaneous breast biopsy procedures, including axillary lymph nodes.6

MammoSTAR® Biopsy Site Marker
The all-natural biopsy marker provides a non-metal marking alternative with a long-lasting beta glucan carrier for unique patient sensitivities.

MammoMARK® & CorMARK® Biopsy Site Identifier
Rapid collagen expansion anchors the marker within the biopsy cavity reducing the likelihood of movement.7,8

LumiMARK™ Biopsy Site Marker
Three multifaceted nitinol shapes designed for easy identification from any angle.9

BiomarC® Biopsy Site Marker
All-natural design in a small size, ideal for superficial lesions.
Ultrasound immediate deployment photo courtesy of Dr. Robin Shermis, ProMedica Breast Care of Toledo
1. HydroMARKTM Device Test – PCR-000414, Summative Usability
2. HydroMARKTM Design Plan – ADD-00013 Rev G, Page 15
3. HydroMARKTM Device Test – PCR-000299, SDR0098 Vacuum Suction Testing
4. HydroMARKTM Device Test – PCR-000305
5. Sakamoto, N., Fukuma, E., Tsunoda, Y. et al. Evaluation of the dislocation and long-term sonographic detectability of a hydrogel-based breast biopsy site marker. Breast Cancer 25, 575–582 (2018). https://doi.org/10.1007/s12282-018-0854-8)
6. Indication may not be approved or available in your region. Please check with your local Mammotome representative.
7. MammoMARK® Device Test – PRC043442 Rev B, pgs. 2, 5-6: Collagen Expansion
8. Corsi, F., Sorrentino, L., Sartani, A., Bossi, D., Amadori, R., Nebuloni, M., Truffi, M., Bonzini, M., & Foschi, D. (2015). Localization of nonpalpable breast lesions with sonographically visible clip: Optimizing tailored resection and clear margins. The American Journal of Surgery, 209(6), 950-958
9. LumiMARK™ Device Tests: PCR-000340 System Design, PCR-000579 Summative Usability, ES-002647 Claims Assessment
Products may not be approved or available in your region. Please check with your local Mammotome representative.