MammoMARK® & CorMARK®
Biopsy Site Identifier
Rapid expansion anchors the marker in place for unsurpassed placement accuracy.1
MammoMARK® & CorMARK® Biopsy Site Identifier
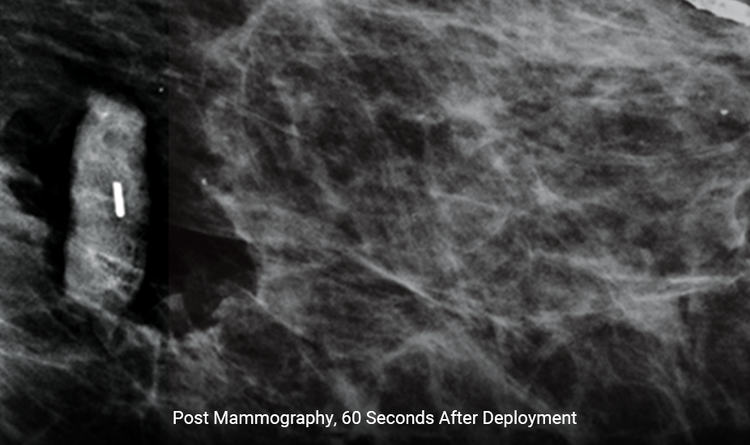
Expands Rapidly
The size increases by 300% within 60 seconds, reducing the likelihood of movement.1,2
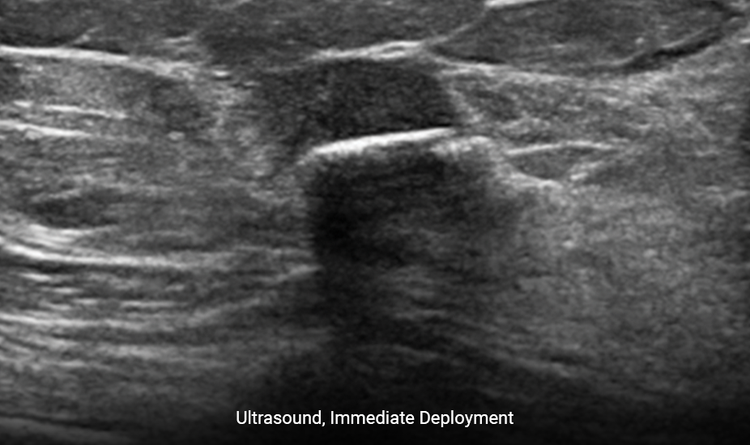
Provides Clear Ultrasound Visibility
The increased surface area provides ease of ultrasound visibility at time of deployment.3
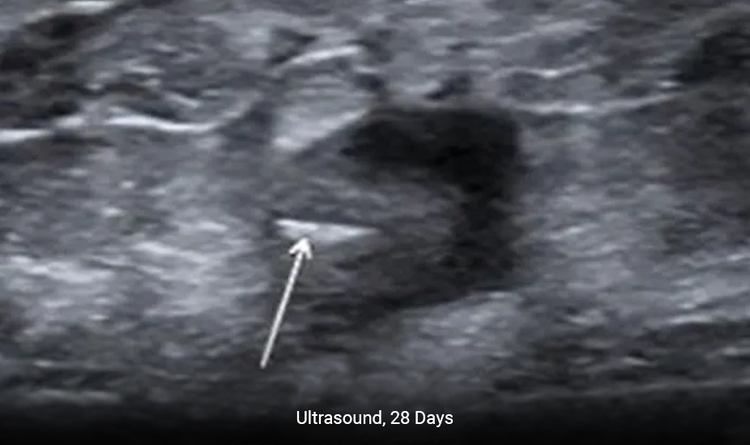
Enables Precise Placement
The MammoMARK® & CorMARK® markers help with accurate placement in the biopsy cavity.3

Absorbs Fluid Quickly
The MammoMARK® & CorMARK® markers assist in fluid management by quickly absorbing surrounding fluid.1
Three distinct shapes for better tracking of multiple biopsy sites
Shapes
- Bowtie
- Triple Twist
- U-Shape



“The most significant advantage we found with the MammoMARK® is the ability to consistently localize [it] using sonography.”
Krakos et al. Advantages of Using the New MammoMark Percutaneous Breast Biopsy Marker – a Large Center Experience.
Frequently Asked Questions
What is the difference between the MammoMARK® & CorMARK® markers?
The applicators are the only difference between the two markers. The CorMARK® markers are the direct puncture applicators and the MammoMARK® markers encompass all others.
What are the MammoMARK® & CorMARK® markers composed of?
The MammoMARK® & CorMARK® markers are composed of bovine collagen and titanium.
Do the MammoMARK® & CorMARK® markers contain any nickel?
No, the markers do not contain any nickel.
Related Products

HydroMARK™ Breast Biopsy Site Marker
Enduring exclusive hydrogel technology provides long-term ultrasound visibility in percutaneous breast biopsy procedures, including axillary lymph nodes.4

HydroMARK™ Plus Breast Biopsy Site Marker
Same great benefits of the original HydroMARK™ markers, with added features designed to alleviate displacement, enhance visibility and ease of locating.5,6,7

MammoSTAR® Biopsy Site Marker
The all-natural biopsy marker provides a non-metal marking alternative with a long-lasting beta glucan carrier for unique patient sensitivities.

LumiMARK™ Biopsy Site Marker
Three multifaceted nitinol shapes designed for easy identification from any angle.8

BiomarC® Biopsy Site Marker
All-natural design in a small size, ideal for superficial lesions.
1. MammoMARK® Device Test – PRC043442 Rev B, pgs. 2, 5-6: Collagen Expansion
2. Corsi, F., Sorrentino, L., Sartani, A., Bossi, D., Amadori, R., Nebuloni, M., Truffi, M., Bonzini, M., & Foschi, D. (2015). Localization of nonpalpable breast lesions with sonographically visible clip: Optimizing tailored resection and clear margins. The American Journal of Surgery, 209(6), 950-958
3. Corsi F, Sorrentino L, Bossi D, Sartani A, Foschi D. Preoperative localization and surgical margins in conservative breast surgery. Int J Surg Oncol. 2013;2013:793819. doi:10.1155/2013/793819
4. Indication for lymph node using HydroMARK™ markers is limited to the United States with other country registrations pending.
5. HydroMARK™ Device Test – PCR-000414, Summative Usability
6. HydroMARK™ Design Plan – ADD-00013 Rev G, Page 15
7. HydroMARK™ Device Test – PCR-000299, SDR0098 Vacuum Suction Testing
8. LumiMARK™ Device Tests: PCR-000340 System Design, PCR-000579 Summative Usability, ES-002647 Claims Assessment
Products may not be approved or available in your region. Please check with your local Mammotome representative.