Mammotome® Markers
A GLOBAL LEADER IN BREAST BIOPSY MARKERS
Offering clinicians the most comprehensive portfolio,
providing a customized solution for every breast biopsy need.
Comprehensive Portfolio. Customized Solutions.
Highly Visible Under all Imaging Modalities1
Whether you are biopsying under stereotactic x-ray, performing a pre-op MRI, or localizing under ultrasound, Mammotome® Markers offer the visibility that you can count on.1
Anti-Migration for Accurate Placement2
We offer markers enclosed by collagen designed to provide anti-migration and hemostatic properties to better assist you with placement accuracy. 2

Compatible With Most Major Breast Biopsy Devices
Mammotome® Markers are designed to work with not only our biopsy devices, but biopsy devices from other companies as well (Hologic® and BD Bard®).
Marker Options for Patient Compatibility
Worried about nickel allergies? Mammotome offers non-nickel breast biopsy marker options.
Breast marking technologies designed to meet your patients’ individual needs.
The Mammotome® Markers portfolio includes:
Learn More About Mammotome® Markers
Our comprehensive marker portfolio

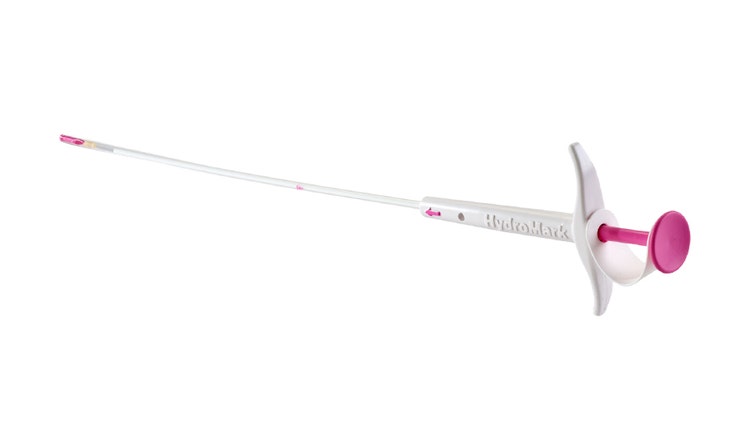
HydroMARK™ Breast Biopsy Site Marker
Enduring exclusive hydrogel technology provides long-term ultrasound visibility in percutaneous breast biopsy procedures, including axillary lymph nodes.3

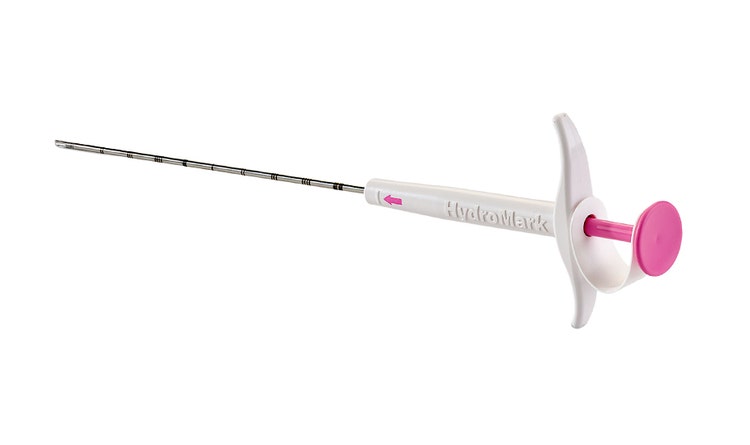
HydroMARK™ Plus Breast Biopsy Site Marker
Same great benefits of the original HydroMARK™ markers, with added features designed to alleviate displacement, enhance visibility and ease of locating.4,5,6

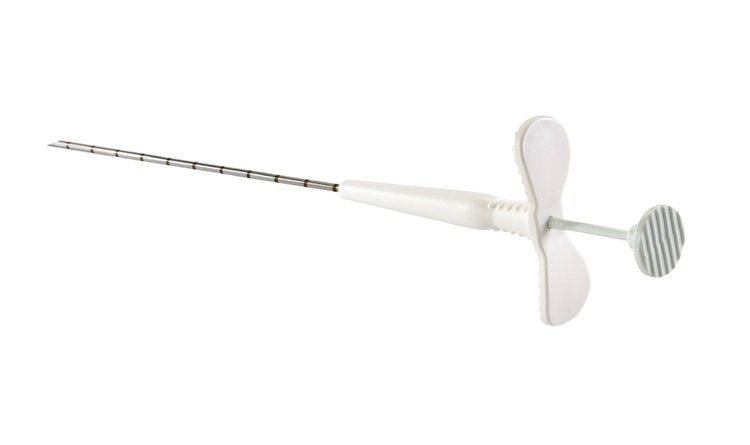
MammoMARK® & CorMARK® Biopsy Site Identifier
Rapid collagen expansion anchors the marker within the biopsy cavity reducing the likelihood of movement.7,8

MammoSTAR® Biopsy Site Marker
The all-natural biopsy marker provides a non-metal marking alternative with a long-lasting beta glucan carrier for unique patient sensitivities.

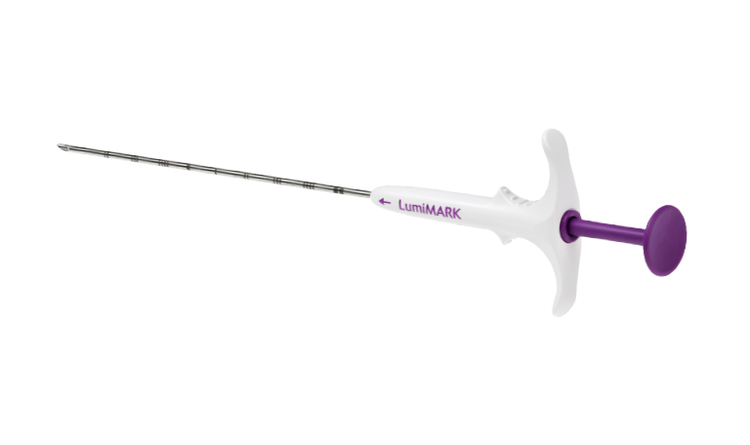
LumiMARK™ Biopsy Site Marker
Three multifaceted nitinol shapes designed for easy identification from any angle.9

BiomarC® Biopsy Site Marker
All-natural design in a small size, ideal for superficial lesions.
Would you like more information about Mammotome® Markers?
FREQUENTLY ASKED QUESTIONS
Is my breast biopsy device compatible with a Mammotome® biopsy marker?
Please review our compatibility brochures available here or speak to your Mammotome representative.
Is it possible to bundle a Mammotome core needle biopsy device with a Mammotome® Marker?
Yes, we do offer bundles with our core needle and breast biopsy markers. Please fill out the Contact Us Form or ask your local Mammotome Breast Care Specialist for more information.
Is it possible to bundle a Mammotome® Elite biopsy device with a Mammotome® Marker?
No, unfortunately we do not offer a bundle deal for our Mammotome® Elite biopsy device and marker products.
RELATED PRODUCTS

Mammotome® Elite System
Combines vacuum-assisted biopsy tissue quality with the ease and speed of a single insertion biopsy device.
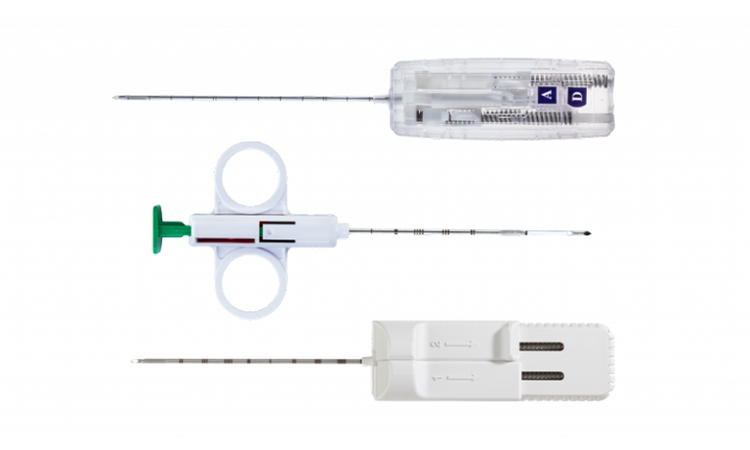
Mammotome® Core Needles
Mammotome® core needles offer clinicians a variety of core needle technologies: semi-automatic, single-stage, and dual-stage devices.

Magseed® Magnetic Marker
A precise 1x5mm marker that becomes detectable when temporarily magnetized by the Sentimag® probe.
1. Pinkney, Journal of Diagnostic Medical Sonography 29(4) 151 – 158, DOI
2. Corsi, The American Journal of Surgery (2015) 209, 950-958
3. Indication for lymph node using HydroMARK™ markers is limited to the United States with other country registrations pending.
4. HydroMARK™ Device Test – PCR-000414, Summative Usability
5. HydroMARK™ Design Plan – ADD-00013 Rev G, Page 15
6. HydroMARK™ Device Test – PCR-000299, SDR0098 Vacuum Suction Testing
7. MammoMARK® Device Test – PRC043442 Rev B, pgs. 2, 5-6: Collagen Expansion
8. Corsi, F., Sorrentino, L., Sartani, A., Bossi, D., Amadori, R., Nebuloni, M., Truffi, M., Bonzini, M., & Foschi, D. (2015). Localization of nonpalpable breast lesions with sonographically visible clip: Optimizing tailored resection and clear margins. The American Journal of Surgery, 209(6), 950-958
9. LumiMARK™ Device Tests: PCR-000340 System Design, PCR-000579 Summative Usability, ES-002647 Claims Assessment
Products may not be approved or available in your region. Please check with your local Mammotome representative.